Nutraceuticals, Cosmeceuticals, Pharma OTC























Sarvotham, a contract development and manufacturing organization (CDMO) and contract manufacturing organization (CMO) with over 25 years of experience, offers comprehensive end-to-end solutions for the healthcare industry. Our expertise encompasses formulation development, Analytical services, Regulatory expertise, Pre-clinical and clinical services and manufacturing excellence.
At Sarvotham, quality is paramount. Commitment to excellence is reflected in ISO 9001 (2000) and EMS 14000 (2004) certifications, ensuring consistent quality management and environmental responsibility.
Partner with Sarvotham to:
Legacy of Quality, Future of Innovation
At Sarvotham, we are committed to providing exceptional care, guided by three core values
Integrity is paramount at Sarvotham. We adhere to the highest ethical and moral principles,
ensuring:
We believe in open communication, clear processes, and accessible information, empowering
clients in their care. This includes:
At Sarvotham, we actively seek and implement effective solutions, going beyond simply
identifying problems. We foster:
Regularly evaluating client satisfaction and using feedback for improvement.



Integrity is paramount at Sarvotham. We adhere to the highest ethical and moral principles, ensuring:
We believe in open communication, clear processes, and accessible information, empowering
clients in their care. This includes:
At Sarvotham, we actively seek and implement effective solutions, going beyond simply
identifying problems. We foster:
Regularly evaluating client satisfaction and using feedback for improvement.
These principles are the foundation of our operations and shape every interaction.
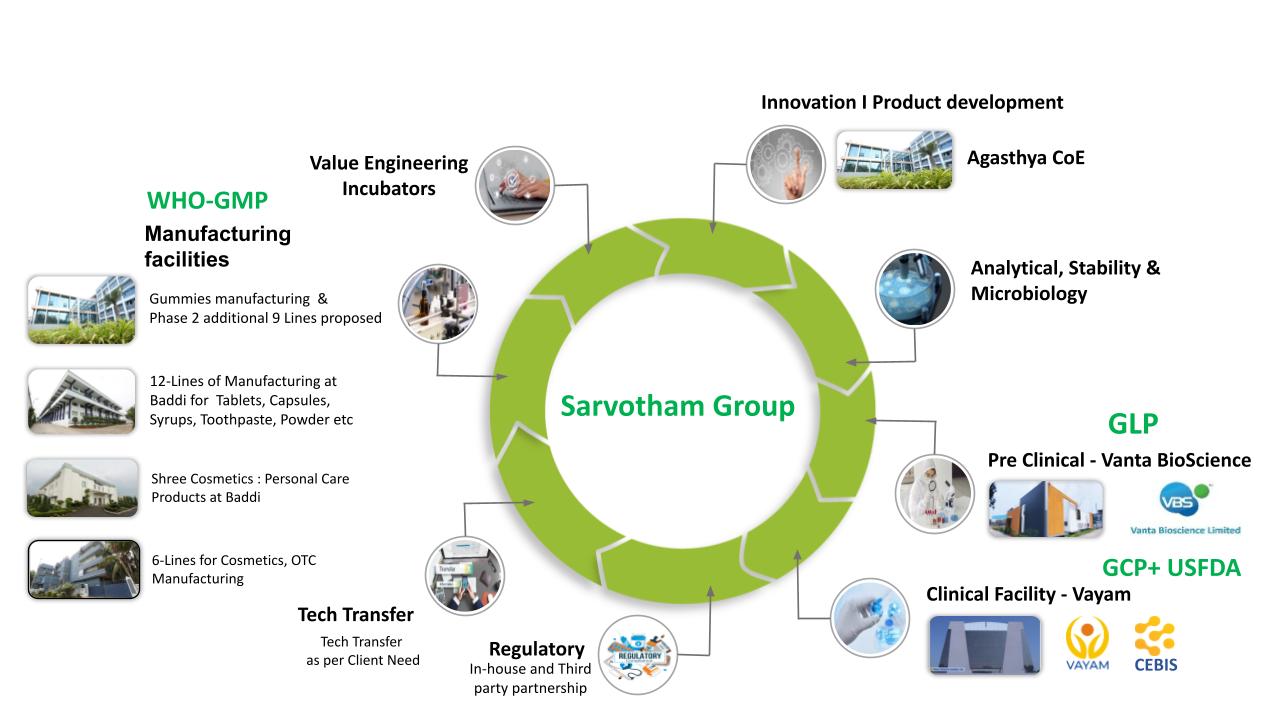
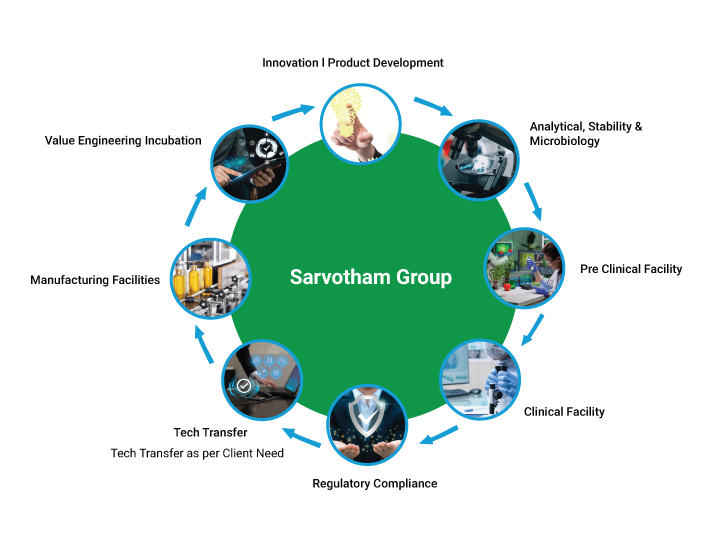
Explore our comprehensive CDMO services and experience the Sarvotham advantage. Together, let’s shape a healthier world
At Sarvotham, we are committed to transforming innovative ideas into tangible healthcare solutions.
Our dedicated research and development team is focused on:
Through rigorous research and development efforts, Sarvotham aim to make a significant impact on the healthcare industry.
At Sarvotham, specialized in transitioning products from the pilot stage to commercial manufacturing.
Experienced team ensures a seamless transition, focusing on:
By providing comprehensive support for pilot-to-commercial manufacturing, we help our clients bring their innovative products to market successfully.
At Sarvotham, employ state-of-the-art analytical techniques to ensure the highest standards of quality, safety, and compliance.
Analytical laboratories are equipped with advanced instrumentation and staffed by experienced professionals who conduct rigorous testing and analysis. Through commitment to analytical excellence, Sarvotham deliver accurate and reliable results that support the development and commercialization of high-quality healthcare products.
At Sarvotham, conducts comprehensive stability studies to validate the shelf life and performance of products. Stability testing protocols are designed to simulate real-world storage conditions and evaluate the product's stability over time.
By conducting thorough stability studies, we ensure that products maintain their integrity and efficacy throughout their intended shelf life.
With facilities in Hyderabad, Baddi, Chennai and Ahemedabad, Sarvotham is well-positioned to serve clients worldwide. Our strategic locations provide accessibility and scalability, ensuring that we can meet the needs of our global customer base.
Key strategies to support global expansion:
Key aspects of our manufacturing excellence:
Clinical Studies are essential for evaluating the safety and efficacy of new healthcare products in humans. These studies provide critical data to support regulatory submissions and ultimately bring products to market.
Key objectives of clinical studies for Sarvotham:
Batch Registration is a critical process in the pharmaceutical industry that ensures the traceability and quality of manufactured products. At Sarvotham, we implement robust batch registration procedures to maintain compliance with regulatory requirements and ensure product safety and efficacy.
Key aspects of batch registration at Sarvotham:
Product Development Goals for Sarvotham:
›Regulatory Expertise is crucial for navigating the complex landscape of healthcare product
development and commercialization. At Sarvotham, our team of regulatory experts possesses the knowledge and experience to ensure compliance with local and international regulations.
Key areas of regulatory expertise at Sarvotham:
Sarvotham adheres to ISO 9001 (2000), EMS 14000 (2004), GMP, and Schedule M (FDA India) standards. Quality is our hallmark.










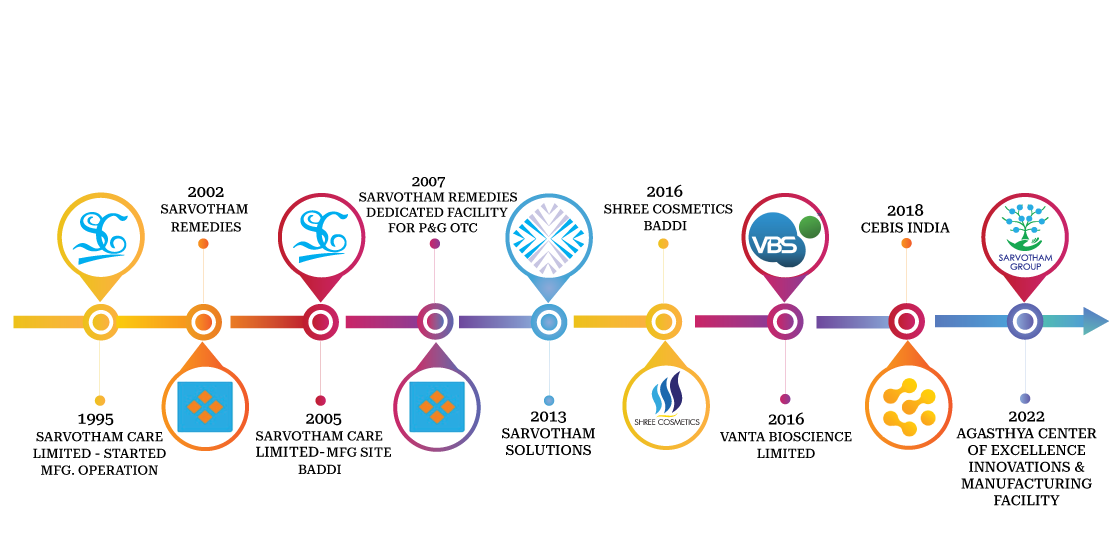
With 25+ years of experience, Sarvotham is a leading Contract Manufacturing (CMO) and Contract Development and Manufacturing Organization (CDMO), offering comprehensive end-to-end solutions. Our expertise spans across clinical services, pre-clinical services and manufacturing excellence.
©2024 Sarvotham, Designed by TheBluIdeas